Waste PC derived degradable epoxy hardener
Prof. Ching-Hsuan Lin, Department of Chemical Engineering
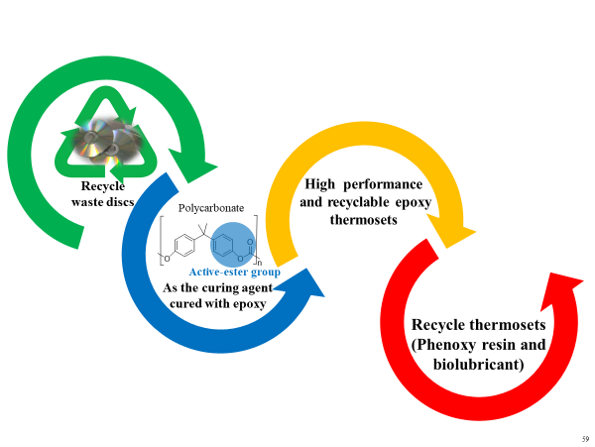
Our laboratory utilizes recycled polycarbonate from discarded optical discs, referred to as Waste Polycarbonate (WPC), as an active ester-type epoxy resin hardener. The WPC-epoxy cured system exhibits comparable thermal properties to commercially available PN/epoxy systems, achieving a 100% atomic utilization efficiency for recycled PC.
Typically, cured epoxy resins cannot be easily degraded and recycled, leading to environmental problem. However, the WPC-epoxy cured system contains degradable functional groups that can solve environmental issues associated with epoxy resins.
The degradation products, such as 1,3-dihexylurea and phenolic resin, have usability, thereby fulfilling the
sustainable objective of reusing waste materials.
Typically, cured epoxy resins cannot be easily degraded and recycled, leading to environmental problem. However, the WPC-epoxy cured system contains degradable functional groups that can solve environmental issues associated with epoxy resins.
The degradation products, such as 1,3-dihexylurea and phenolic resin, have usability, thereby fulfilling the
sustainable objective of reusing waste materials.
Development of Green Catalysts
Prof. Chi-Tien Chen, Department of Chemistry
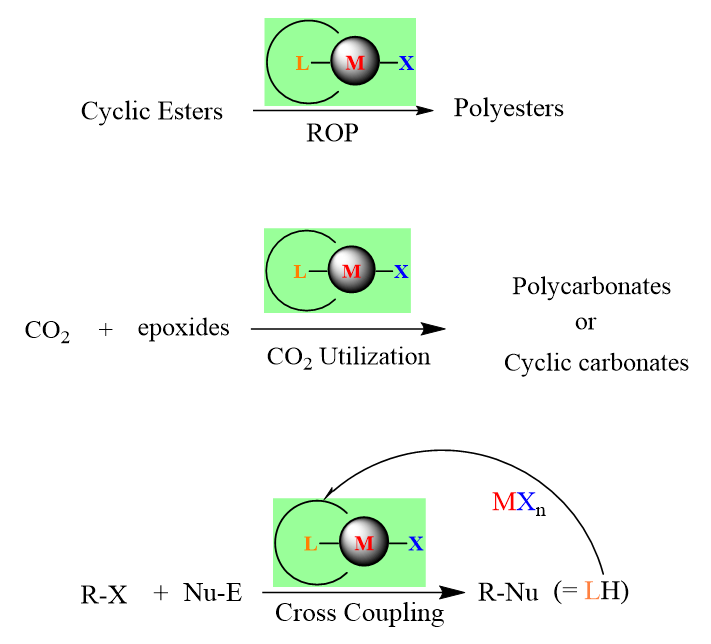
Our current interests are mainly focusing on the development of metal complexes for applications in various catalytic reactions. The major interests are:
(a)Ring-opening polymerization of ε-caprolatone and lactide.
(b) Coupling or copolymerization of CO2/epoxides
(c) Cross coupling reactions
(a)Ring-opening polymerization of ε-caprolatone and lactide.
(b) Coupling or copolymerization of CO2/epoxides
(c) Cross coupling reactions
Development of Green Catalyst
Prof. Dong-Sheng Lee, Department of Chemistry
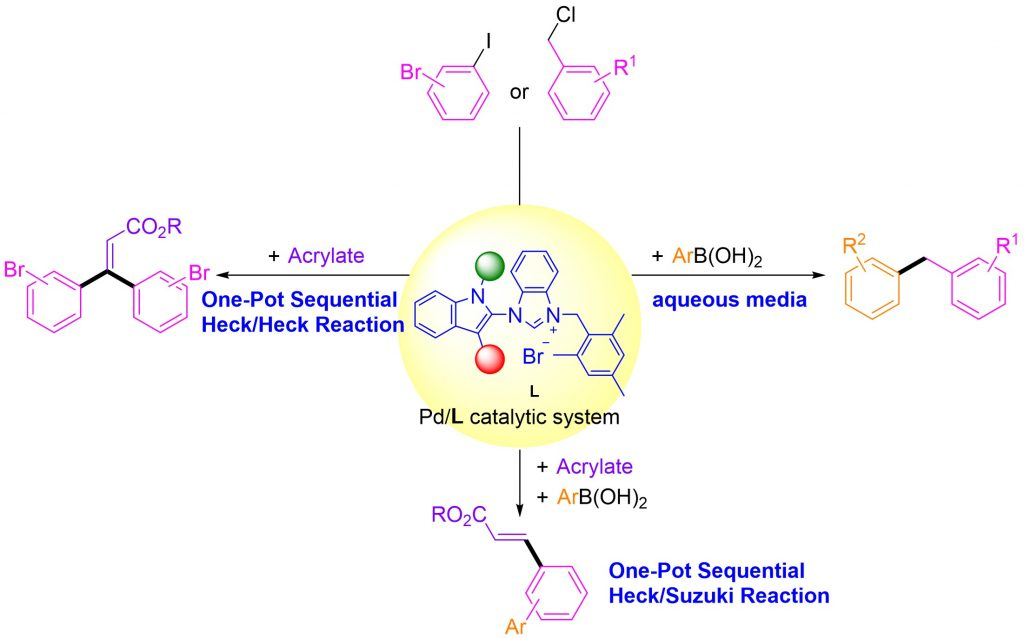
Our research team has successfully developed nitrogen-containing heterocyclic carbenoid precursors with an indole skeleton. The structure of these precursors is modifiable and can efficiently form catalysts with palladium under alkaline conditions. These catalysts can achieve carbon-carbon bond coupling at low loading (0.25 or 0.5 mol%).
(The results have been published in Asian Journal of Organic Chemistry.)
The water-soluble nature of these precursors allows the catalysts formed with palladium to catalyze carbon-carbon bond formation reactions in aqueous environments, showcasing great potential as green catalysts. Furthermore, the preparation of these precursors requires only three simple steps, using cost-effective starting materials, making them valuable for large-scale production.
(The results have been published in Asian Journal of Organic Chemistry.)
The water-soluble nature of these precursors allows the catalysts formed with palladium to catalyze carbon-carbon bond formation reactions in aqueous environments, showcasing great potential as green catalysts. Furthermore, the preparation of these precursors requires only three simple steps, using cost-effective starting materials, making them valuable for large-scale production.
Development of Mild and Effective Catalyst Alum
Prof. Shun-Yuan Luo, Department of Chemistry
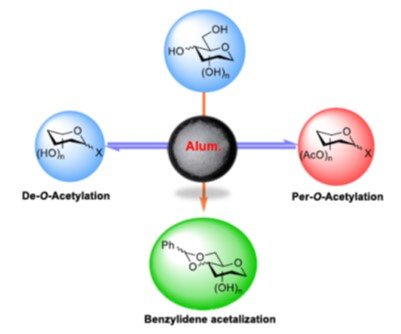
Our research team has optimized the per-O-acetylation, deacetylation, and benzylidene protection of carbohydrates and investigate the scope of saccharides via alum-catalysis. The study expanded to investigate the per-O-acetylation of different saccharides, including galactose and mannose, which produced stereoisomeric mixtures. This study investigated the generality of the de-O-acetylation method on a variety of carbohydrates using per-O-acetyl saccharide substrates bearing different functional groups. The direct protection of benzylidene in addition to acetylation/de-O-acetylation with a focus on optimizing reaction conditions for the synthesis of carbohydrate derivatives. Overall, the study offers insights into optimizing deacetylation, per-O-acetylation, and benzylidene protection investigating the scope of saccharides via alum-catalysis.
(The results have been published in Asian J. Org. Chem.)
(The results have been published in Asian J. Org. Chem.)
Low Carbon Green Chemical Processes
Prof. Cheng-Kun Lin, Department of Chemistry
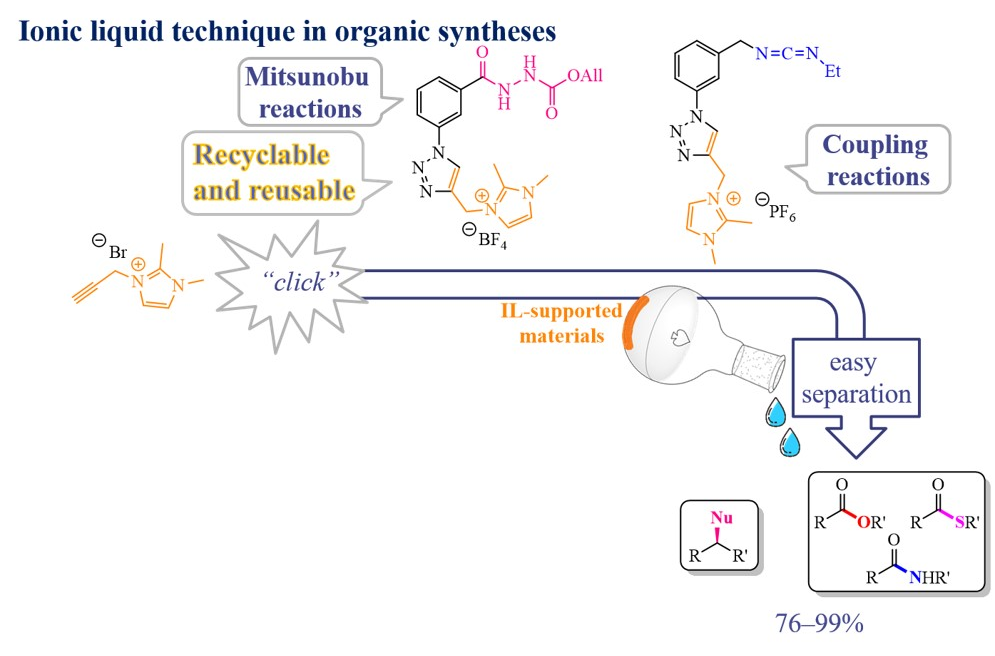
The research team, under the leadership of Dr. Cheng-Kun Lin, has introduced an innovative method for developing enhanced reagents utilizing ionic liquids. By employing traditional thin-layer chromatography techniques, they can monitor the advancement of homogeneous organic synthesis supported by ionic liquids. Typically, achieving high-purity molecules doesn′t require column chromatography; instead, a straightforward washing of crude mixtures is sufficient. Furthermore, the capability to regenerate and reuse by-products, supported by ionic liquids, elevates the value and eco-friendliness of this approach. Ionic liquid-supported organic synthesis expedites the swift and simultaneous production of small molecules with diverse structures.